Texas.If you buff Harry Potter novels and movies, you might already be familiar with the cloak that could make Harry Potter "disappeared". Now, the cloak is not just a fantasy. A team of researchers at the University of Texas at Austin created the robe.
Andrea Lu creation cloak could make objects invisible despite only being able to be applied in light of microwaves.

MOSCOW: The number of fighters of the fifth generation Sukhoi T-50 PAK will be increased to 14 units from now on only three. Russia decided to increase the number of the most technologically advanced aircraft in the world to test fly deep.
Russian Air Force Commander Alexander Zelin on Monday, according to RIA Novosti, said, "There have been three fighters who took part in the flight test, the other three expected to be tested in the near future. Entire number of aircraft for flight test 14 units."
There is a lot of effort to make the smartphone as a productivity device like a PC. The effort comes as more powerful smartphone capabilities and increasingly dependent person with that device.
One smartphone vendor who wants that is BlackBerry. The Canadian company also expects its new mobile phone that can simplify the user's life so no need to carry an additional device.
News of mini-sized DSLR Canon turned out to be true. Camera EOS 100D is officially named the company introduced Thursday (3/21/2013) ago.
The camera is in the United States sold under the name "Rebel SL1 'is claimed to be the smallest and lightweight DSLR. Physical dimensions 117x91x69mm, and weighs 407 grams including the battery.
Google decided to shut down Google Reader news reading service on 1 July 2013. This is an act of "clean-up" for Google to focus on its core business.
Google's surprising decision this makes Google Reader users looking for an alternative similar service. This moment is also used by the other news reader service providers, to attract new users to migrate from Google Reader.
Here's an alternative service similar to Google Reader:

Enchanting. That first impression when I saw the HTC Butterfly. Her whole screen is dark, with a rosy color cast on the back, presenting a promising elegance.
However, design is not just created to pamper the eyes, but also determine the comfort when using these smart mobile phone. She is the entrance to attract anyone.
Smart phone design is really slick. In addition to unsightly, HTC Butterfly has sides ergonomically friendly. There's no corners were jerking the surface of the hand.
Seed plants have flowers. Interest in plant seeds serve as generative reproduction. In the flowers are male and female genitals. Male genitals in a flower seed plants consisting of a stamen (stamens). Has stamen anthers (anthera) that is located at the end of stalk juice (filament). In anthers with one or more space juice (puzzle) which is the formation of pollen (pollen). Pollen is called the male gamete. Genitals are female (pistil) can be composed of ovary (ovarian) stalk buds (stillus), and head Pistil (stigma). Will the fruit is on the plant seeds as the presence of ovules. Plant ovary lies at the base of flower seeds (reseptakel) and shape bubble.
John Dalton was a British teacher. He put forward the theory of atoms based on the Law of Conservation of Mass (proposed by Antonie Lavoiser) which states that in a reaction, the reaction mass before and after the same are subscription and Comparative Law Fixed (presented by Josepht Proust) which states that in a pure chemical substance, the ratio of the mass of the element elements in each compound are fixed. For example, is made up of 2 parts water and 1 part H O to form H2O or 1 gram H vs 8 grams of O. Where else would we get water composed of elements with the same ratio.
Dalton's atomic theory includes four (4) as follows:
Each chemical element arranged on small particles that can not be broken again called atoms. Atoms can not be created nor destroyed during a chemical change. Atoms are round like a ball.
Constituent atoms of an element are identical in mass (weight) and certain properties. However, atoms of one element differ from the atoms of other elements.
When forming compounds, different elements joined by a simple comparison. For example, an atom A with an atom B (AB), or one atom A to atom B two (AB2), and so on.
A chemical reaction is merely a displacement of atoms from one set to the other combinations. Atoms themselves individually and always remains unchanged.
Dalton's atomic theory gives details and a more complete explanation than Democritus statement. But this theory is also not perfect. Why is that? The answer please see Atomic Model: JJ Atomic Theory Thomson.
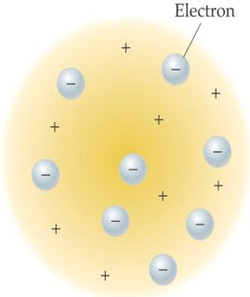
Physicist Joseph John Thomson (1856-1940) was a scientist who was born in Cheetham Hill, where at that place anyway Thomson was named professor of experimental physics since 1884. The study did result in the discovery of the electron Thomson. He knew that the gas is a substance that is able to deliver electricity. Thomson also one of the pioneers of nuclear physics. Thomson won the Nobel Prize for physics in 1906.
This article is a continuation of the previous discussion as well as to answer questions in writing Atomic Model: Atomic Theory Dalton. Dalton's atomic theory long held by scholars at that time until the discovery of negatively charged electrons by JJ Thomson in 1897 (see History of discovery in Proton, Neutron, and Electron). The discovery of electrons finally broke Dalton theory that atoms are the smallest matter. Because the negativity charged electrons Thomson thinks that there is a positive charge as a counterweight. Thus the atom is neutral.
Thomson atomic model illustrates that the atom was a positively charged sphere. Meanwhile, the electrons (the negatively charged atoms) are spread evenly on the surface of the ball. Negative charges are spread out like raisins in raisin bread. The number of positive charges equal to the amount of negative charge so that the atom is neutral.
The number of positive charges = Number of negative charge
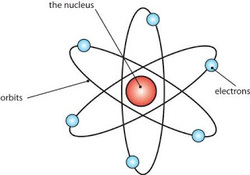
Ernest Rutherford was a chemist who received the Nobel Prize in 1908. He investigated about solving elements and radioactive materials. In 1911, Rutherford with two students (Geiger and Ernest Marsden) has been experimenting with shooting a thin layer of gold using alpha particles, which are known as alpha-ray scattering experiments.
The results of this experiment successfully completed teacher Rutherford atomic theory, namely JJ Thomson. Rutherford model of the atom proposed as follows:
Most of the mass and the whole positive charge contained in an atom is concentrated in a very small region called the nucleus. Atom itself is mostly a blank space.
The amount of positive charge is different from one atom to another atom.
The number of electrons around the nucleus of an atom is like the positive charge on the nucleus. Atom as a whole is neutral.








 RSS Feed
RSS Feed