Ernest Rutherford was a chemist who received the Nobel Prize in 1908. He investigated about solving elements and radioactive materials. In 1911, Rutherford with two students (Geiger and Ernest Marsden) has been experimenting with shooting a thin layer of gold using alpha particles, which are known as alpha-ray scattering experiments.
The results of this experiment successfully completed teacher Rutherford atomic theory, namely JJ Thomson. Rutherford model of the atom proposed as follows:
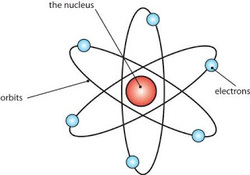
Most of the mass and the whole positive charge contained in an atom is concentrated in a very small region called the nucleus. Atom itself is mostly a blank space.
The amount of positive charge is different from one atom to another atom.
The number of electrons around the nucleus of an atom is like the positive charge on the nucleus. Atom as a whole is neutral.
The results of this experiment successfully completed teacher Rutherford atomic theory, namely JJ Thomson. Rutherford model of the atom proposed as follows:
Most of the mass and the whole positive charge contained in an atom is concentrated in a very small region called the nucleus. Atom itself is mostly a blank space.
The amount of positive charge is different from one atom to another atom.
The number of electrons around the nucleus of an atom is like the positive charge on the nucleus. Atom as a whole is neutral.
 RSS Feed
RSS Feed