Physicist Joseph John Thomson (1856-1940) was a scientist who was born in Cheetham Hill, where at that place anyway Thomson was named professor of experimental physics since 1884. The study did result in the discovery of the electron Thomson. He knew that the gas is a substance that is able to deliver electricity. Thomson also one of the pioneers of nuclear physics. Thomson won the Nobel Prize for physics in 1906.
This article is a continuation of the previous discussion as well as to answer questions in writing Atomic Model: Atomic Theory Dalton. Dalton's atomic theory long held by scholars at that time until the discovery of negatively charged electrons by JJ Thomson in 1897 (see History of discovery in Proton, Neutron, and Electron). The discovery of electrons finally broke Dalton theory that atoms are the smallest matter. Because the negativity charged electrons Thomson thinks that there is a positive charge as a counterweight. Thus the atom is neutral.
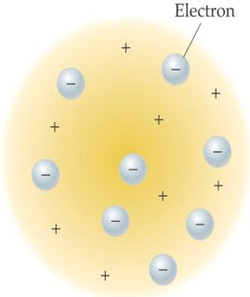
Thomson atomic model illustrates that the atom was a positively charged sphere. Meanwhile, the electrons (the negatively charged atoms) are spread evenly on the surface of the ball. Negative charges are spread out like raisins in raisin bread. The number of positive charges equal to the amount of negative charge so that the atom is neutral.
The number of positive charges = Number of negative charge
This article is a continuation of the previous discussion as well as to answer questions in writing Atomic Model: Atomic Theory Dalton. Dalton's atomic theory long held by scholars at that time until the discovery of negatively charged electrons by JJ Thomson in 1897 (see History of discovery in Proton, Neutron, and Electron). The discovery of electrons finally broke Dalton theory that atoms are the smallest matter. Because the negativity charged electrons Thomson thinks that there is a positive charge as a counterweight. Thus the atom is neutral.
Thomson atomic model illustrates that the atom was a positively charged sphere. Meanwhile, the electrons (the negatively charged atoms) are spread evenly on the surface of the ball. Negative charges are spread out like raisins in raisin bread. The number of positive charges equal to the amount of negative charge so that the atom is neutral.
The number of positive charges = Number of negative charge
 RSS Feed
RSS Feed